Background: Cytokine storm is a complex syndrome driven by hyperinflammation leading to multi-organ dysfunction and high mortality rates. Patients with hematological malignancies are susceptible to different types of cytokine storm syndromes (CSS) including those driven by their disease (e.g., malignancy-associated hemophagocytic lymphohistiocytosis (MA-HLH)), treatment toxicities (e.g., cytokine release syndrome (CRS) related to CAR-T) or infections (e.g., COVID associated cytokine storm). The precise nature of the inflammatory mechanisms of different CSS and their impact on clinical outcomes are poorly understood.
Methods: We conducted a retrospective study to compare the inflammatory marker profiles and survival outcomes of three CSS: CAR-T CRS, MA-HLH and COVID-CS. The cloud-based Foundry platform was used to aggregate and integrate data from patients with hematological malignancies admitted at the MD Anderson Cancer Center between March 2020 and November 2022. Patients in the CAR-T CRS and MA-HLH cohorts met ASTCT Consensus Grading criteria and HLH-2004 criteria respectively. While there are no well-established diagnostic guidelines for COVID-CS, patients in the COVID-CS cohort had laboratory values higher than the upper limit of normal for at least two of C-reactive protein (CRP), Interleukin 6 (IL-6) or Ferritin within 28 days of COVID diagnosis (Table 1). Biomarker datasets were analyzed through regression models and feature selection to determine inflammation patterns across the three cohorts. Survival outcomes were compared across the cohorts and tested for association with biomarkers to determine their prognostic relevance (Figure 1).
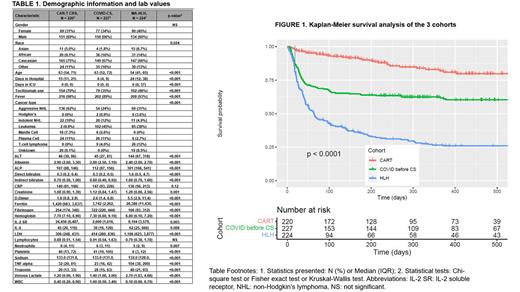
Results: A total of 671 in-patients met inclusion criteria across the three patient cohorts: CAR-T CRS (220), MA-HLH (224) and COVID-CS (227). The proportions of different disease types differed among the cohorts (Table 1) with aggressive B-cell lymphomas representing the largest group in the CAR-T CRS cohort (62%) whereas leukemia was the most frequent diagnosis in the COVID-CS (45%) and MA-HLH (38%) cohorts. While fever and fatigue were common presenting symptoms of all 3 CSS, the frequencies of more severe symptoms such as hypotension and capillary leak syndrome were increased in patients with MA-HLH. The median length of hospitalization differed significantly (p < 0.001) across the cohorts: MA-HLH (24 days), CAR-T CRS (15 days) and COVID-CS (6 days). The MA-HLH cohort had the highest proportion of patients requiring ICU admissions and the highest levels of inflammatory markers such as D-dimer, ferritin, IL-6, IL-2 soluble receptor and TNF-alpha and the lowest levels of fibrinogen and albumin. Clustering analysis of laboratory values revealed substantial overlap in patterns between the COVID-CS and CAR-T CRS cohorts, but the MA-HLH cohort was a more distinct cluster. Patients with CAR-T CRS had significantly longer survival (p < 0.0001) than either COVID-CS (hazard ratio 3.7) or MA-HLH (hazard ratio 8.1); MA-HLH patients had the shortest overall survival (Figure 1). These differences were robust after adjusting for differences in baseline demographics. Univariate regression modelling revealed many of the laboratory values to be significantly associated with survival; multivariable analysis found ferritin, LDH and platelet count to be the most significant predictors. Administration of tocilizumab did not improve survival in COVID-CS or MA-HLH.
Conclusions: In this retrospective, single-institution study of 3 different types of CSS, we observed that patients with hematological malignancies exhibit different symptom patterns at clinical presentation, have differing inflammatory biomarker profiles and experience variable clinical outcomes depending on the underlying etiology of the CSS. Patients with CAR-T CRS had significantly better survival outcomes compared to those with COVID-CS and with MA-HLH; the latter group had the worst outcomes among the 3 cohorts. This study provides insights into the hyperinflammatory profiles of different CSS in patients with hematological malignancies and highlights the need for developing effective treatments to improve patient outcomes.
Disclosures
Strati:Hutchinson MedoPharma: Consultancy; Astrazeneca Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche Genentech: Consultancy; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; ALX Oncology: Research Funding. Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. Westin:Calithera: Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; MonteRosa: Consultancy; Nurix: Consultancy; SeaGen: Consultancy; Abbvie: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding; Kymera: Research Funding. Khawaja:Merck: Research Funding; Medscape: Honoraria. Nastoupil:Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AstraZeneca: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Mulanovich:SOBI: Consultancy; Nkarta: Consultancy; Legend Biotech: Consultancy. Daver:Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Agios: Consultancy; Jazz: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Amgen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Hanmi: Research Funding; Trovagene: Research Funding; FATE: Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; AROG: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Celgene: Consultancy; Kronos Bio: Research Funding. Flowers:Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics Jansen: Consultancy; SeaGen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Acerta: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Jannsen Pharmaceuticals: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Neelapu:Athenex: Consultancy, Other: Advisory board member; Fosun Kite: Consultancy, Other: Advisory board member; Allogene: Consultancy, Other: Advisory board member, Research Funding; Morphosys: Consultancy, Other: Advisory board member; Astellas Pharma: Consultancy, Other: Advisory board member; Caribou: Consultancy, Other: Advisory board member; Bluebird Bio: Consultancy, Other: Advisory board member; Bristol Myers Squibb: Consultancy, Other: Advisory Board Member, Research Funding; Adicet Bio: Consultancy, Other: Advisory board member, Research Funding; Incyte: Consultancy, Other: Advisory board member; Sana Biotechnology: Consultancy, Other: Advisory board member, Research Funding; Kite, A Gilead Company: Consultancy, Other: Advisory Board Member, Research Funding; Sellas Life Sciences: Consultancy, Other: Advisory board member; Merck: Consultancy, Other: Advisory Board Member; Janssen: Consultancy, Other: Advisory board member; Chimagen: Consultancy, Other: Advisory board member; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; Orna Therapeutics: Consultancy, Other: Advisory board member; Takeda: Consultancy, Other: Advisory board member; Synthekine: Consultancy, Other: Advisory board member; Carsgen: Consultancy; Precision Biosciences: Research Funding; Longbow Immunotherapy: Current holder of stock options in a privately-held company; N/A: Patents & Royalties: Related to cell therapy and the safety switch described (intellectual property). Iyer:Legend: Research Funding; CRISPR Therapeutics: Research Funding; Spectrum: Research Funding; CureBio: Honoraria; Salarius Pharmaceuticals, Inc.: Consultancy; Takeda: Research Funding; Rhizen: Research Funding; Merck: Research Funding; Yingli: Consultancy, Research Funding; Affimed: Research Funding; Innate: Research Funding; Myeloid: Research Funding; Target Oncology: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal